E. coli pathogenesis
- Bacterial and host factors in development of extraintestinal Escherichia coli infections in pigs and chickens
- Characterization of the attaching and effacing mechanism in Escherichia coli of animal origin
1. Bacterial and host factors in development of extraintestinal Escherichia coli infections in pigs and chickens
We demonstrated a role for Type 1 (Figure 1) and P fimbriae in the early colonization of the upper respiratory tract and later systemic stages of infection, respectively, during development of E. coli septicemia in chickens (Pourbakhsh et al., 1997a; Pourbakhsh et al., 1997b). We also confirmed that P fimbriae mediate bacterial adherence to pig polymorphonuclear leukocytes but inhibit the oxidative response of these cells, thus probably contributing to bacterial survival (Ngeleka and Fairbrother, 1999). We contributed in showing that the temperature sensitive hemagglutinin Tsh is involved in the development of E. coli aerosacculitis in chickens (Dozois et al, 2000). By using appropriate mutants, we showed that O78 lipopolysaccharide and the K1 capsule but not curli, type 1, or P fimbriae contributed to bacterial resistance to serum and colonization of internal organs by avian pathogenic E. coli (Mellata et al, 2003a). Using these same mutants, we confirmed a role for type 1 fimbriae in promotion of initial phagocytosis, but also in the protection of bacteria from subsequent killing by heterophils (Mellata et al, 2003b) (Figures 2a, 2b). Our results also indicated a role for K1 capsule, O78 antigen, and P fimbriae in initial avoidance of phagocytosis, and an additional role for O78 antigen, and P fimbriae in subsequent protection against the bactericidal effects of phagocytes. Further studies suggested that the non-specific host immune response, as evidenced by production of nitric oxide (NO), oxidative burst and the induction of inflammatory cytokines in macrophages, is important in the initial steps of avian pathogenic E. coli infection and that certain bacterial virulence factors, such as LPS, Type 1 and P fimbriae modulate this response (Mellata and Fairbrother, 2003).
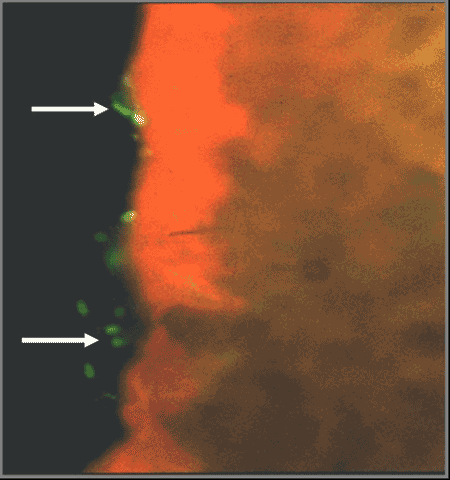
Figure 1. Expression of F1 fimbriae on APEC bacteria colonizing the trachea of a chicken
Reference
Dozois CM, Chanteloup N, Dho-Moulin M, Brée A, Deautels C and Fairbrother JM (1994). Bacterial colonization and in vivo expression of F1 (Type 1) fimbrial antigens in chickens experimentally infected with pathogenic Escherichia coli. Avian Diseases 38: 231-239.
Figures 2a, 2b. Association and viability of APEC strains and various mutants in contact with heterophils and macrophages
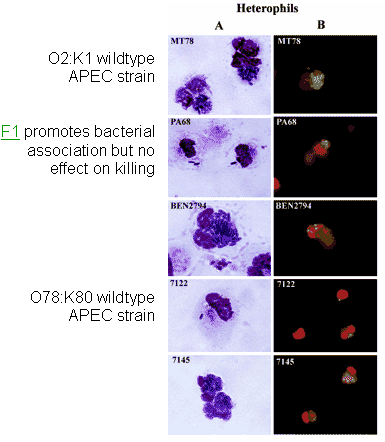
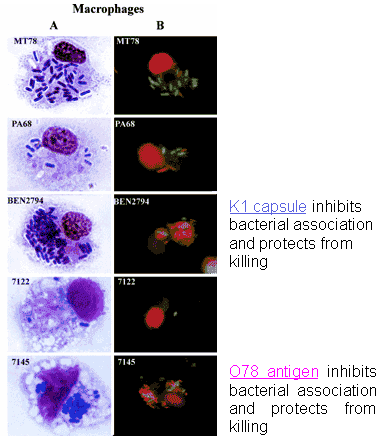
Reference
Mellata et al., 2003b
We had previously demonstrated an increased expression of the inflammatory cytokines Il-1, Il-12, and TNF in the intestinal mucosa of newborn germ-free pigs orally challenged with an opportunistic E. coli strain and manifesting septicemia (Fournout et al., 2000). We have now shown that weaned pigs, following oral administration of the mycotoxin fumonisin B1, become more susceptible to infection by opportunistic E. coli, demonstrating a decreased expression of cytokines Il-1, Il-6, Il-8, and Il-12 in the intestinal mucosa (Oswald et al., 2003; Oswald et al., 2000). Thus, we provide evidence for one environmental factor which may increase host susceptibility to infection by opportunistic E. coli, probably through an alteration of the local cytokine response.
References
Dozois CM, Dho-Moulin M, Brée A, Fairbrother JM, Desautels C and Curtiss III R (2000). Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli, and localization and analysis of the tsh genetic region. Infection and Immunity 68: 4145-4154.
More details
Fournout S, Dozois CM, Odin M, Desautels C, Peres S, Herault F, Daigle F, Segafredo C, Laffitte J, Oswald E, Fairbrother JM and Oswald IP (2000). Lack of a Role of cytotoxic necrotizing factor 1 toxin from Escherichia coli in bacterial pathogenicity and host cytokine response in infected germfree piglets. Infection and Immunity 68: 839-847.
More details
Mellata M and Fairbrother JM (2003). Phagocyte response to avian pathogenic E. coli infection in vitro. 103rd General Meeting of American Society for Microbiology, Washington D.C., Virginie, USA. (Abstract).
Mellata M, Dho-Moulin M, Dozois CM, Curtiss III R, Brown PK, Arné P, Brée A, Desautels C and Fairbrother JM (2003a). Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infection and Immunity 71: 536-540.
More details
Mellata M, Dho-Moulin M, Dozois CM, Curtiss III R, Lehoux B and Fairbrother JM (2003b). Role of avian pathogenic Escherichia coli virulence factors in bacterial interaction with chicken heterophils and macrophages. Infection and Immunity 71: 494-503.
More details
Ngeleka M and Fairbrother JM (1999). F1651 fimbriae of the P fimbrial family inhibit the oxidative response of porcine neutrophils. FEMS Immunology and Medical Microbiology 25: 265-274.
More details
Oswald IP, Fournout S, Laffitte J, Odin M and Fairbrother JM (2000). Dietary fumonisin B1 exposure increases pig susceptibility to opportunistic Escherichia coli infection and decreases local inflammatory cytokine production. In proceedings of the 16th Congress of the International Pig Veterinary Society, Melbourne, Australia, p.57.
Oswald IP, Desautels C, Laffitte J, Fournout S, Péres SY, Odin M, Le Bars P, Le Bars J and Fairbrother JM (2003). Mycotoxin fumonisin B1 increases intestinal colonization by pathogenic Escherichia coli in pigs. Applied Environmental Microbiology 69: 5870-5874.
More details
Pourbakhsh SA, Dho-Moulin M, Brée A, Desautels C, Martineau-Doizé B and Fairbrother JM (1997a). Localization of the in vivo expression of P and F1 fimbriae in chickens experimentally inoculated with pathogenic Escherichia coli. Microbial Pathogenesis 22: 331-341.
More details
Pourbakhsh SA, Boulianne M, Martineau-Doizé B, Dozois CM, Desautels C and Fairbrother JM (1997b). Dynamics of Escherichia coli infection in experimentally inoculated chickens. Avian Diseases 41: 221-233.
More details
Financial Support
The Conseil des recherches en pêche et en agroalimentaire (CORPAQ), the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Fonds Québecois de la Recherche sur la Nature et les Technologies (FQRNT).
2. Characterization of the attaching and effacing mechanism in Escherichia coli of animal origin
We have recently focused our research program on the study of host and bacterial factors involved in the pathogenesis of attaching and effacing E. coli infections in pigs, an ongoing theme of our laboratory for the last 10 years (An et al., 1999; An et al., 2000; DesRosiers et al., 2001; Batisson et al., 2003; An et al., 1997; Zhu et al., 1996; Zhuet al., 1995a; Zhu et al., 1995b; Zhu et al., 1994). We had previously characterized the intimin adhesin of attaching and effacing E. coli (AEEC) associated with postweaning diarrhea in pigs and demonstrated a role for this protein in the development of attaching and effacing (AE) lesions, using pig ileal in vitro organ culture (IVOC) (Zhu et al., 1995a; Zhu et al., 1995b; Zhu et al., 1994). We have recently identified a novel bacterial factor, Paa, which appears to be involved in the early steps of the adhesion mechanism of AEEC (Batisson et al., 2003). Using pig intestinal IVOC, we showed that chicken egg yolk antibodies specific for intimin, Tir, the bacterial receptor for intimin, and Paa significantly reduced adherence of a wide range of AEEC from humans and other animal species (Batisson et al., 2003; Girard et al., 2003), strengthening the argument for a role of these factors in the AE process. We then demonstrated that intimin subtype influences intestinal adherence and tropism of AEEC strains in pig intestinal IVOC and that a tir mutant of an AEEC strain demonstrates close adherence to the epithelial cells of porcine intestinal IVOC segments, with microvillous effacement but with no evidence of actin polymerization and pedestal formation (Figures 3 and 4), and that intimin and EspA, a constituent of the type III secretion apparatus, seem to be involved in this phenotype (Girard et al., 2005a). This study provides further evidence for the existence of a host cell-encoded intimin receptor, in addition to the bacterially encoded Tir.
Figure 3. Wild-type human EPEC strain E2348/69 adheres to small intestinal epithelial cells and induces typical A/E lesions in porcine ileal IVOC (in vitro organ culture) segments
Reference
Girard F, Batisson I, Frankel GM, Harel J and Fairbrother JM (2005). Interaction of enteropathogenic and Shiga-Toxin producing Escherichia coli with porcine intestinal mucosa: role of Intimin and Tir in adherence. Infection and Immunity 73: 6011 Fig. 5.
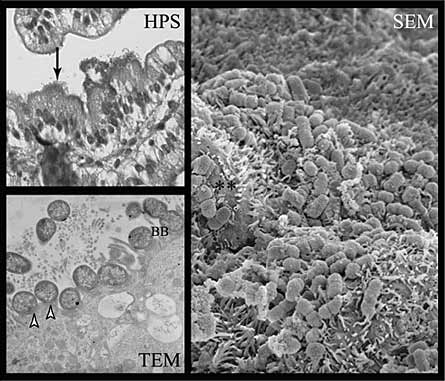
Figure 4. E2348/69Δtir mutant strain shows reduced adherence capacity to small intestinal epithelial cells on HPS-stained sections (HPS, arrow), although a loss of A/E capacity, but a close attachment with effacement of the brush border (**) with no evidence of actin polymerization and pedestal formation (arrowheads) on TEM and SEM sections
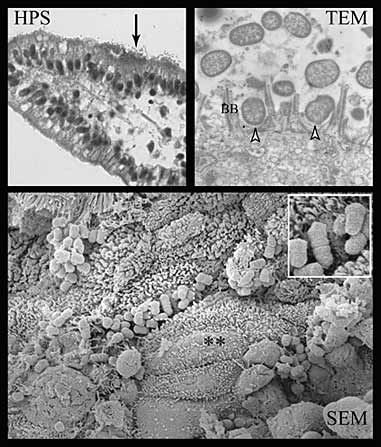
Reference
Girard F, Batisson I, Frankel GM, Harel J and Fairbrother JM (2005). Interaction of enteropathogenic and Shiga-Toxin producing Escherichia coli with porcine intestinal mucosa: role of Intimin and Tir in adherence. Infection and Immunity 73: 6012 Fig. 6.
Although we have been able to reproduce AE lesions in newborn piglets (Zhu et al., 1994), until recently, we had been unable to induce such lesions in older conventional pigs with a more mature immune system. We have now successfully reproduced extensive AE lesions in weaned pigs treated with dexamethasone, used in immunosuppressive doses (Girard et al., 2005b). We demonstrated that cytokines IL-1, IL-6, IL-8, and IL-12 are significantly upregulated in the ileum of challenged pigs not treated with dexamethasone, whereas dexamethasone inhibits such upregulation. Our results confirm that the host immune status is a key determinant protecting against the development of AE lesions in weaned pigs, and it appears that IL-1, IL-6, IL-8, and IL-12 are involved in the natural resistance to development of these lesions.
References
An H, Dubreuil JD, Fairbrother JM and Harel J (1997). Cloning and characterization of the Eae gene from a dog. FEMS Microbiology Letters148: 239-245.
More details
An H, Fairbrother JM, Dubreuil JD and Harel J (1999). Cloning and characterization of the esp region from a dog attaching and effacing Escherichia coli strain 4221 and detection of EspB protein-binding to HEp-2 cells. FEMS Microbiology Letters 174: 215-223.
More details
An H, Fairbrother JM, Desautels C, Mabrouk T, Dezfulian H and Harel J (2000). Presence of the LEE (locus of enterocyte effacement) in pig attaching and effacing Escherichia coli and characterization of eae, espA, espB, and espD genes of PEPEC (pig EPEC) strain 1390. Microbial Pathogenesis 28: 291-300.
More details
Batisson I, Guimond MP, Girard F, An H, Zhu C, Oswald E, Fairbrother JM, Jacques M and Harel J (2003). Characterization of the novel factor paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infection and Immunity 71: 4516-4525.
More details
DesRosiers A, Fairbrother JM, Johnson R, Desautels C, Letellier A and Quessy S (2001). Phenotypic and genotypic characterisation of Escherichia coli verotoxin-producting isolates from humans and pigs. Journal of Food Protection 64: 1904-1911.
More details
Girard F, Batisson I, Harel J and Fairbrother JM (2003). Use of Egg Yolk-Derived Immunoglobulins as an Alternative to Antibiotic Treatment for Control of Attaching and Effacing Escherichia coli Infection. 103rd General Meeting of American Society for Microbiology, Washington D.C. Virginie, USA. (Abstract).
Girard F, Batisson I, Frankel G, Harel J and Fairbrother JM (2005a). Interaction of enteropathogenic and Shiga-Toxin producing Escherichia coli with porcine intestinal mucosa: Role of Intimin and Tir in adherence. Infection and Immunity 73: 6005-6016.
More details
Girard F, Oswald I, Taranu I, Hélie P, Appleyard G, Harel J and Fairbrother JM (2005b). Host immune status influences the development of attaching and effacing lesions in weaned pigs. Infection and Immunity 73: 5514-5523.
More details
Zhu C, Harel J, Jacques M, Desautels C, Donnenberg MS, Beaudry M, and Fairbrother JM (1994). Virulence properties and attaching-effacing activity of Escherichia coli O45 associated from swine post weaning diarrhea. Infection and Immunity 62: 4153-4159.
More details
Zhu C, Harel J, Dumas F and Fairbrother JM (1995a). Identification of EaeA protein in the outer membrane of attaching and effacing Escherichia coli O45 from pigs. FEMS Microbiology Letters 129: 237-242.
More details
Zhu C, Harel J, Jacques M and Fairbrother JM (1995b). Interaction with pig ileal explants of Escherichia coli from swine with post weaning diarrhea. Canadian Journal of Veterinary Research 59: 118-123.
More details
Zhu C, Ménard S, Dubreuil JD and Fairbrother JM (1996). Detection and localization of the EaeA protein of attaching and effacing Escherichia coli O45 from pigs using a monoclonal antibody. Microbial Pathogenesis 21: 205-213.
More details
Financial Support
This work was supported by grants from the Medical Research Council of Canada (MRC), the Conseil des recherches en pêche et en agroalimentaire du Québec (CORPAQ), the Natural Sciences and Engineering Research Council (NSERC) of Canada, the Fonds Québecois de la Recherche sur la Nature et les Technologies (FQRNT), a Programme de soutien aux initiatives internationales de recherche et d'innovation (PSIIRI) of the Ministère du développement économique et régional et de la recherche (MDERR) du Québec grant, by Gestion Univalor, limited partnership grant, by the Fonds du Centenaire of the Faculté de médecine vétérinaire of the Université de Montréal, and by a European Commission grant.
 Top of page
Top of page





